FAQs
The FAQs serve as a guide and support for reflection and are subject to change. Each project is assessed in the light of its specificity and context, and only the decision of the cureg is binding.
01. What should I do if I want to change a project that has already been approved by the Committee?
We must be informed of any changes to a project already approved by the Committee (end date, number of participants, procedure, etc.) before the project’s end date. You can get the relevant form by emailing commission-ethique@unige.ch and providing the following information:
- Applicant’s full name
- The project title
- The project number, as indicated on the initial approval form
02. How much does it cost to have a project assessed?
This service is currently free of charge.
03. If I’m involved in a research project that has already been approved by another research ethics committee, do I still need to submit the project to CUREG2.0?
Decisions by other university or public research ethics committees are usually recognized by CUREG2.0. However, we may require you to take additional steps, so you should contact CUREG2.0 before collecting data or carrying out any other procedures.
04. How do I ensure my study’s participants can provide free and informed consent?
4.1 Written consent
As part of the consent process, you may use a pre-study consent form (CF). This should allow participants to make an informed decision about whether they want to participate in the study. In general, a good way to assess your CF is to ask yourself if the person participating in your study might be surprised by aspects of your protocol that they might not expect. If so, then your CF is likely incomplete. Even if the CF informs the person that some things will not be revealed until the end, they should still be warned about what they are about to do.
CFs are typically comprised of the following parts:
- The general purpose of the research activity.
- Required information in order to fulfil the activities (filling out questionnaires, conducting tests, etc.).
- The duration, cost, and potential gain (compensation, gift certificate, etc.).
- Potential risks and how they are controlled by the research protocol.
- The anonymous or non-anonymous nature of the data being collected.
- Whether personal data or sensitive personal data are collected, and if so, which ones.
- Where the data goes (storage and duration of storage, anonymization, etc.), its uses and access rights, deletion, or rectification.
- The individuals who will have access to personal data.
- The researchers’ contact information.
The researchers will determine whether further clarification is necessary. Examples of consent forms and a guide to writing a consent form are available for download by clicking the links below:
CUREG-2023-06-20-GuideRedactionFormulaireConsentment
CUREG-2023-06-20-InformationConsentement_adultes
CUREG-2023-06-20-InformationConsentement_parents-enfants
4.2 Oral consent
In the social sciences and humanities, and particularly in the context of qualitative methods using interviews and/or ethnographic observations, providing written consent can sometimes be problematic or not appropriate. These methods are based on a situated and relational approach with regards to the interaction between the researcher and participant. The expression “investigative relationship” points to a social relationship, and written consent can disrupt this relationship by introducing misunderstandings or preventing the development of mutual trust. This is particularly true when researchers and participants are in regular contact in an ethnographic field (“in situ”).
Furthermore, written consent is deemed not appropriate when participants have characteristics specifically related to literacy or the contractual dimension of the signature, or when the researcher adopts an immersive and/or participatory ethnographic approach.
In cases where written consent is problematic or irrelevant, the applicant to the CUREG must be accompanied by an information and consent protocol in lieu of the written information and consent form. There are two options:
a) mixed written + oral: participants are given a research information sheet (a written document), they are asked questions about the research and their participation, and then are asked to provide oral consent.
b) entirely oral: participants are informed orally, they are invited to ask questions about the research and their participation and are then invited to give their oral consent (e.g., individual oral information in the case of individual interviews; oral information during information sessions at the beginning of the ethnographic fieldwork in a community, etc.).
The information and consent protocol must include the desired option (a or b) and provide details about how it is to be implemented.
05. What preventive measures should be put in place for items related to suicidal ideations?
Including questions on suicidal ideations e.g., item 9 of the Beck Depression Inventory (BDI), is considered potentially problematic because the individual may declare suicidal thoughts or ideas, thus involving the ethical responsibility of the researchers (Green et al., 2015).
If suicidal ideations have been identified, researchers must refer the individual to the appropriate health authorities[1] (for a discussion of this issue see Hom et al., 2017). The solution recommended by the committee is to remove these questions (e.g., use the BDI excluding item 9). If this interferes with the research objectives, an intervention protocol must be created. See the model proposed by Carleton University
Examples are also available in Bailey et al. BMC Medical Ethics, 2020
Response anonymity or confidentiality does not absolve researchers of this responsibility. Where appropriate, a procedure for anonymization must be put in place after problematic cases have been identified. If those responsible for the research choose this option, the consent form should be modified. If the procedure does not allow for the identification of individuals (MTurk, Prolific, etc.), it would be appropriate to automate the receipt or display of an alert to the individual before they exit the application.
If your protocol does not directly address suicide, the solution recommended by the CUREG is to remove questions about suicidal ideations from the questionnaires or to use a measurement tool that does not contain a question related to suicide.
References
Bailey, E., Mühlmann, C., Rice, S. et al. Ethical issues and practical barriers in internet-based suicide prevention research: a review and investigator survey. BMC Med Ethics 21, 37 (2020). DOI: 10.1186/s12910-020-00479-1
Green, K. L., Brown, G. K., Jager-Hyman, S., Cha, J., Steer, R. A., & Beck, A. T. (2015). The predictive validity of the beck depression inventory suicide item. The Journal of clinical psychiatry, 76(12), 1683-1686.
Hom et al., 2017. Ethical issues and practical challenges in suicide research collaboration with institutional review boards. Crisis, 38, 107-114. DOI: 10.1027/0227-5910/a000415
[1] HUG Psychiatry Dept (Urgences secteur psychiatrique)
HUG Young Adults Psychiatry Dept (Unité psychiatrie du jeune adulte)
HUG Young Adult Health (Santé jeunes)
06. I am measuring depression using clinical scales, what are the recommendations?
Including a measure of depression in a protocol always poses an ethical problem (e.g., Clark et al., 2003; https://doi.org/10.1053/apnr.2003.50003). Researchers must therefore ensure that this measure is necessary. Depression scales (e.g., CES-D or BDI) are not diagnostic (Sheehan et al., 2013; https://dx.doi.org/10.1186%2F1472-6939-14-4) and are not designed to measure the intensity of a depressed mood. If the goal is to obtain an estimate of participants’ mood, the committee suggests using tools that specifically measure mood, such as the PANAS.
If the purpose of the research clearly justifies the use of a depression scale, it should not be used alone to suggest to participants a suspected diagnosis of depression. If a participant’s score is above the recommended cut-off, a recommendation can be made to seek advice from their physician. The committee recommends providing participants with a list of accessible centres (thus, depending on their location) and an informative brochure on depression.
The committee would like to draw the attention to the researchers regarding the risk of worrying participants without reason. It is recommended that a high criterion be used before alerting the participant. Certain scales (e.g., the CES-D) must be linked to the presence of other illnesses that may artificially increase the score on this scale.
References:
Clark, P. C., & Dunbar, S. B. (2003). Identifying possible depression in clinical research: Ethical and outcome considerations for the investigator/clinician. Applied Nursing Research, 16(1), 53-59. DOI: 10.1053/apnr.2003.50003
Sheehan, A. M., & McGee, H. (2013). Screening for depression in medical research: ethical challenges and recommendations. BMC medical ethics, 14(1), 1-4. DOI: 10.1186%2F1472-6939-14-4
07. What precautions should I take before using Zoom for a research project?
- UNIGE’s Zoom license must be used.
- The use of Zoom implies the collection of personal data, and you must therefore inform the participants appropriately via the consent form.
- We also invite you to read the document “Good practices in terms of security and confidentiality on Zoom”, available on the university’s website (https://www.unige.ch/enseignement-a-distance/files/4916/3542/4864/11._Zoom11_Securite__confidentialite_v3.1.pdf) and to apply them to your research practices.
- It is not permitted to share the Zoom link of a meeting via social networks.
- If you wish to record interviews via Zoom, a local recording should be used, not a cloud recording.
- Since Zoom does not allow for local audio recordings that are independent of the recording of the image appearing on the camera, it is advisable, if the quality of the recording is sufficient, to use a dictaphone (or any other external recorder) if only an audio recording is planned. Another possibility is to inform the participant that the recording is audio-visual, but that only the audio recordings will be kept and analysed within the research framework (.mp3 files), while the audio-visual recordings will be destroyed immediately (.mp4 files) following local file generation.
- Participants should be asked to change the name that appears on their window before the meeting begins.
- The consent form cannot be sent/collected by Zoom.
In any case, it is necessary to include this additional information in the consent form if testing is to be done virtually. We suggest using the following text:
“If health conditions require it, we will conduct the interviews/tasks/other [to be adapted according to your case] using Zoom software under license with the University of Geneva. The use of Zoom involves the collection of personal data. The Zoom platform is certified by the Swiss-US Privacy Shield agreement. The meeting link will be sent to you by e-mail (your e-mail address will be deleted as soon as we no longer need it) [to be adapted according to your procedure]. Prior to the session, please replace the name on your Zoom display window with a nickname/participant code that we have provided for you [choose the appropriate option for you], so that your identity remains hidden. You will be notified when the audio/video recording begins [if applicable; choose the applicable option for you], and you will be asked to confirm your agreement to the recording verbally. The recording will be stored locally (not on the cloud) and used for strict research purposes. [if only audio recording is needed, specify that audio-visual recordings will be destroyed immediately following the interview].”
08. Virtual reality and risk of cybersickness: what to do?
Ethical question of concern: endangering participants
Immersion in virtual reality can trigger undesirable effects that are well documented in the literature and are referred to as cybersickness. The symptoms are similar to those of motion sickness (kinetosis); those who suffer from motion sickness are more likely to suffer from cybersickness. For this reason, the CUREG recommends that exclusion criteria for those who suffer from kinetosis (self-reported sensitivity to motion sickness) be applied in virtual reality research.
Symptoms of motion sickness are rarely severe and are of short duration. For example, vomiting is rare (less than 15 per 1000).
The symptoms observed systematically in a portion of the population (estimated at 20% in the first 30 minutes) are, in decreasing order of intensity: 1) disorientation (dizziness, imbalance); 2) eye problems (eye fatigue, blurred vision, headaches), 3) nausea (heartache, dizziness).
These risks are reduced in situations where protective factors are present:
– include a negligible delay in the synchronization of the head movements and the movements of the virtual scene (it is recommended to use the most advanced equipment possible, including computers and graphic cards).
– include few rapid movements in the scene (such as scrolling through a landscape).
– age: the literature on the effect of age is sparse, but most existing studies indicate that cybersickness decreases sharply in adulthood, with some authors even indicating that it is non-existent after age 50 (Mousavi et al, 2013).
Nevertheless, even when taking this into account, it must be estimated that between 0 and 20% of participants are likely to experience cybersickness, which justifies the use of a control and support device.
With respect to monitoring, cybersickness can be detected by observing the person’s behaviour (agitation, non-response, etc.), by self-reports, or by a single-item measurement performed regularly (at the end of the habituation phase and then at regular intervals). The task must be interrupted above a predetermined threshold.
In accordance with the literature, the severity of the cybersickness will be moderate at worst. The person should be immediately removed from the helmet and remain seated for at least 30 minutes (average time for symptoms to disappear reported in the literature), and at least until their self-assessment on the single-item measure has dropped to a very low level. In cases of more severe symptoms, the procedures recommended for first aid will be applied.
Reference:
Mousavi, M., Jen, Y. H., & Musa, S. N. B. (2013). A review on cybersickness and usability in virtual environments. In Advanced Engineering Forum (Vol. 10, pp. 34-39). Trans Tech Publications Ltd. DOI: 10.4028/www.scientific.net/AEF.10.34
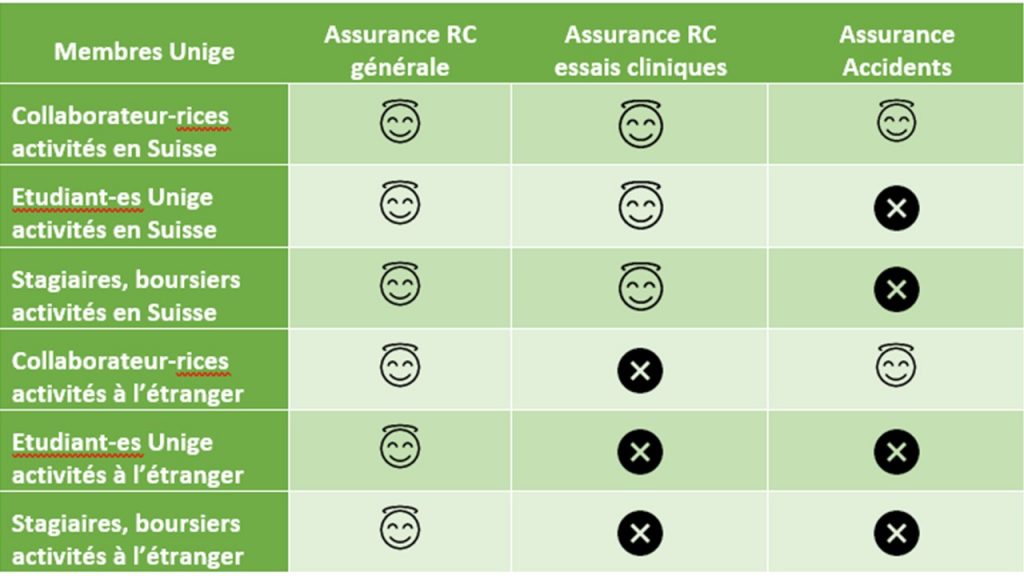
09. Research and Civil Liability Insurance
9.1 What civil liability (CL) insurance coverage(s) is there for Unige researchers?
There are two types of Civil Liability (CL) insurances at the University of Geneva.
- A general CL insurance that covers all “standard” activities of the members of the university, whether they are students, PAT, or PENS collaborators. The activities concerned are those related to the mission of the university, namely teaching, research or services to the community.
- A special CL insurance, called “clinical trials” This is an insurance policy whose benefits are extended to research activities for which risks have been identified by a committee that has assessed the project, such as the CCER or the CUREG. Historically, this CL insurance was put in place for research that included clinical trials, hence its name.
9.2 How are research projects covered by “clinical trials” CL insurance identified?
Research projects for which risks have been identified are reported to the “clinical trials CL” insurance every trimester. Risks that are mainly related to the physical or psychological health of participants and/or researchers are of concern. The activities of all persons involved in the project are covered by the “clinical trials CL” insurance, as long as these activities take place in Switzerland.
9.3 Are students conducting research as part of a bachelor’s, master’s or continuing education project covered by Unige’s general CL insurance?
Yes, students carrying out research work during a bachelor’s, master’s or continuing education program are covered by Unige’s general CL insurance for their research activities when they take place in Switzerland, as is the case for any member of the University of Geneva, provided that they are registered at the university.
9.4 Are students carrying out research involving risks related to the physical or psychological integrity of participants and/or researchers covered by the Unige’s “clinical trials CL” insurance?
Yes, if projects involving students are declared to the “clinical trials CL” insurance by the CUREG following the project’s thorough evaluation procedure.
9.5 How are the activities of those who do not have a contract with Unige covered? We are referring to interns, scholarship holders or doctoral students without remuneration.
A clause stipulates that persons are insured “in the performance of their duties for UNIGE.” The scope of the CL insurance is thus broad. However, an agreement must be formalized between the UNIGE and the scholarship holder or volunteer in connection with the research duties, typically by registration with the university or through an internship contract.
9.6 Does the general CL insurance cover activities carried out abroad?
Yes, the CL insurance is also valid for research activities performed outside Switzerland.
9.7 Does the “clinical trials CL” insurance cover activities carried out abroad?
No, the research activity must take place in Switzerland to be covered.
9.8 Does this CL insurance cover activities carried out in Geneva on participants located abroad?
No, for legal reasons. Research activities carried out by researchers located in Geneva but using participants recruited on European or international platforms will not be covered by the CL insurance. However, if participants come to UNIGE to participate in the research activity, these activities will be covered because Swiss law prevails. Please note that the simple fact of having a questionnaire filled out on foreign territory is not covered by our CL insurance.
10. Research and Accident Insurance (“Assurance Accident”)
10.1 What ACCIDENT INSURANCE coverage is provided for researchers employed by Unige?
Unige employees benefit from the Accident Insurance provided by Unige in line with their contract. This coverage ends upon termination of their employment or service.
10.2 If employees carry out their research abroad, do they still benefit from these two types of insurance, accident, and CL?
Yes, for accident insurance, yes for the “general” CL insurance and no for the “clinical trials” CL insurance. For the latter, it is the “host” institution that provides coverage for the part of the research it conducts, and the coverage must include the students involved.
10.3 What about accident coverage for researchers without a contract but affiliated to Unige (typically master’s students, doctoral or post-doctoral fellows, or volunteers)? What recommendation can be made?
Students should seek personal coverage. The DIFE distributes this information to new students. If they work at least 8 hours per week, their employer will pay for the coverage.
In summary:
11. My research protocol involves home visits to participants. What are CUREG’s recommendations?
For some projects, it is necessary to visit participants’ homes. This can sometimes pose a risk for the researcher. The research supervisor must prioritise the protection of the individuals, especially the students or young researchers, who make the home visit. The CUREG will therefore always carefully examine protocols involving home visits. The CUREG recommends that home visits only be considered if the protocol requires them (and there is no equivalent alternative). In this case, if it does not interfere with the objectives or feasibility of the research, the following aspects may be considered:
- making the visit in pairs.
- the researcher knows the start and end time of the visit.
- those who make the visit are able to send a message to notify the end of the visit.
- If the visit lasts longer than expected, the supervisor must act accordingly.
- those who make the visits are aware of what to do in case of any problem.
12. Preparatory studies for research involving human participants: do I need to apply to CUREG2.0 for certification?
Preparing a research project often requires testing a device on a small number of people, to evaluate its feasibility or to validate part of the material. These tests can also be used to get a feel for the field, to identify potential participants, etc. UNIGE does not require researchers to request a CUREG certificate prior to data collection as part of a preparatory study for a research project. However, this study must be conducted with a small number of participants and is not to be published as the main study in a scientific article. It is explicitly associated with a larger research project. The fact that a certification is not required does not absolve those responsible from respecting the ethical principles of research involving human participants.
13. If I collect personal data as part of my research, what should I look out for?
Preamble:
The university law does not provide a legal basis for the processing of research data and therefore the Law on Public Information, Access to Documents, and Protection of Personal Data (LIPAD) applies to the activities of the university (and therefore to its employees). This cantonal law governs the processing of personal data, sensitive personal data, or the establishment of personality profiles in the context of a research project, except for research projects that fall within the scope of the Federal Act on Research involving Human Beings (HRA) and that are submitted to the Cantonal Research Ethical Committee (CCER).
Consequently, all research projects that do not fall within the scope of the HRA and that involve the collection of non-anonymous data are affected by the LIPAD.
Personal data is defined as any piece of information or group of information that allows for the unequivocal identification of a natural or legal person under private law, such as the identity, date of birth, postal address, e-mail address, etc.
Sensitive personal data and personality profiling are a specific category of personal data that require additional steps. For more details, please refer to the FAQ n° 15.
By processing, we mean (art. 4; letter e – LIPAD): “any operation relating to personal data – regardless of the means and procedures used – in particular the collection, storage, use, modification, communication, archiving or destruction of data.”
Thus, a certain number of principles must be respected, here we list the main ones:
- Principle of proportionality (art. 36 LIPAD): you may only collect personal information that is useful for your purposes: for example, if you need the age but the exact date of birth (01.01.1962) is not useful, then you should not ask for it.
- Purpose principle (art. 35 al. 1 LIPAD): you must tell your participants what information you want to collect about them and the purpose of its collection i.e., why you are collecting this information. This must be done at the time of collection of this information. You may use the data collected ONLY for the purposes originally stated to the participants.
- Principle of security (art. 37 LIPAD): you are obliged to protect this information against any unlawful processing and to ensure its confidentiality by putting in place appropriate protective measures.
- The principle of the right to forget (destruction of data – art. 40 LIPAD): as soon as this information is no longer necessary for your purposes, you must destroy For example, as soon as the name or contact information of a person is no longer useful, this information must be deleted from your files, notes, documents, etc.
Since November 16, 2021, it is no longer necessary to declare files containing personal data to the cantonal Data Protection and Transparency Officer (PPDT). Thanks to the actions taken by UNIGE’s DPO[1] in consultation with UNIGE’s legal affairs department and the CUREG, a generic declaration of files to the catalogue from the cantonal authority is now sufficient for all research projects evaluated by the CUREG and in which personal data are processed. The PPDT has agreed given that the controls relating to the processing of personal data in a research context carried out by the CUREG and the DPO are well supervised.
[1] Alain Jacot-Descombes was appointed as UNIGE’s Data Protection Officer (DPO) in April 2021.
14. What is meant by sensitive personal data or personality profiling within the context of the LIPAD?
According to article 4 (b and c), the law defines these notions as follows
Sensitive personal data: “personal data on religious, philosophical, political or trade union opinions or activities, health, intimate sphere or ethnicity, social assistance measures, criminal or administrative proceedings or sanctions.”
For example, if you ask a mother, what illness her child has, then you are processing (collecting) sensitive personal data. In other words, if you collect the diagnosis(es) (dyslexia, autism, diabetes, etc.) of a person (adult or minor) then you are processing (collecting) sensitive personal data.
Here is another example: if, in the context of a research project, you ask people about their religious practices, you are also processing (collecting) sensitive personal data under the LIPAD.
Consequence: an authorization request to the State Council (Conseil d’Etat) is necessary (see FAQ 15).
Personality profiling: “a collection of data allowing an assessment of the essential characteristics of a natural person.”
This notion remains vague and is not very operational. We sought to clarify this definition with the DPO, who sent us the definition of this concept in the new Federal Act on Data Protection (DPA – Article 5, letters f and g, dated June 7, 2022, this text has not yet been implemented):
- “f) profiling: any form of automated processing of personal data consisting in using such data to evaluate certain personal aspects relating to a natural person, particularly to analyse or predict aspects concerning work performance, economic situation, health, personal preferences, interests, reliability, behaviour, location or moving of the physical person.
- g) high-risk profiling: any profiling that entails a high risk to the personality or fundamental rights of the person of concern, since it leads to matching of data that allows the essential characteristics of the personality of a physical person to be assessed.”
For research, the CUREG considers that a research project deals with personality profiling when tools (quantitative or qualitative) are used that are based on scientific approaches to personality traits, and that allow the respondent to be described on several fundamental dimensions of their personality (e.g., profiling on the “Big Five” dimensions). In this case, the CUREG will invite the person in charge to contact the DPO to check with them whether steps with the State Council (Conseil d’Etat) are necessary (see FAQ 15). Measures of attitudes towards oneself (e.g., self-esteem), values (e.g., solidarity), or single personality traits (e.g., extraversion) are excluded.
15. What steps should I take if I collect sensitive personal data/personality profiles as defined by the LIPAD throughout the course of my research?
Reminder: for data to be considered sensitive personal data/personality profiling, the data must be processed in a non-anonymous way i.e., associated with personal data. For anonymous research (starting from the point of data collection), there is no sensitive personal data nor personality profiling.
The legislator has considered that sensitive personal data/personality profiles are information deserving of a higher level of protection, with the sole aim of protecting the fundamental rights of the individual.
Thus, according to article 41, paragraph 1, letter f of the LIPAD, it is necessary to obtain an authorization from the State Council (Conseil d’Etat) prior to processing sensitive personal data (SPD) or personality profiling. As a reminder, data collection is already considered by the law as a type of processing.
The authorisation request from the State Council must be done as soon as the research project has been accepted by the CUREG.
-
- Procedure:
- From the CUREG’s perspective:
- If a project is concerned by this procedure, the CUREG informs the research supervisor and asks them if they can transmit the revised version to the DPO so that he can verify anything related to the protection of personal data corresponds to the legal rules. He may even ask for modifications regarding solely this issue.
- From the research supervisor’s perspective:
- Based on the model provided by CUREG, the research supervisor drafts a letter addressing the State Council.
- To draft this letter, we invite you to contact Mr. Alain Jacot-Descombes, DPO of the University of Geneva (pdt@unige.ch).
- The person in charge will be responsible for transmitting any useful documents to the State Chancellery.
- The research supervisor will receive a decision from the State Council.
- From the CUREG’s perspective:
- Procedure:
- Special cases:
- If you collect your data anonymously, such as in the following cases:
- Distribution of an online questionnaire, access to anonymous or anonymised databases
- Setting up a procedure that ensures that at no time can you link participants’ responses to their identity
- In these cases, it will not be necessary to apply for authorisation from the State Council for the processing of sensitive personal data/personality profiling within the LIPAD’s framework since you will at no time be aware of the identity of the participants.
- If you collect your data anonymously, such as in the following cases:
16. I am collecting personal data, but my research takes place abroad. Do I still need to follow the LIPAD’s regulations?
The answer is yes.
In this context, it is not the site of the data collection that determines whether LIPAD regulations apply. It is the fact that the data is processed and collected by UNIGE employees that determines whether this law applies. Thus, if you collect sensitive personal data or conduct personality profiling, it will be necessary to take steps with the State Council (Conseil d’Etat) (see FAQ 15).
17. Using online platforms
What is a “cloud” platform?
- An online computer service offered via the internet by a service provider outside UNIGE to carry out certain operations necessary for research. These operations can consist of data collection, storage or analysis, participant recruitment, or online experiments.
What are the security criteria to watch out for when choosing a “cloud” platform for my research?
- The level of sensitivity of the information that will be collected or processed. (Criteria for information classification are available at: https://www.unige.ch/cybersecurite/politiques/classification-de-linformation, only available in French)
- The site of the provider and data host: a Swiss provider and host are recommended. If this is not possible, additional compliance measures will be required in accordance with the document available at: (LINK TO BE CONFIRMED).
- The contractual and technical terms of implementation. (The detailed list of these can be found at: https://www.unige.ch/cybersecurite/pour-les-it-people/regles-minimales-de-securite/solutions-cloud-saas, only available in French)
What are the ethical criteria related to the use of “cloud” platforms?
These criteria can be assessed by asking the following questions about:
- Necessity: is the research unable to be conducted using one of the IT solutions tested and/or offered free of charge by the institution?
- Fair compensation: is the compensation adapted to the standard of living and does not constitute a remuneration? Is a geographical targeting possible in order to adapt the compensation to the local standard of living? Is it customary practice in the given field of research?
- Research transparency: are the purposes and methods of data processing clear? Is consent obtained in the same way as it is for “offline” research?
- Participant supervision: are the necessary guidance and support procedures put in place for sensitive issues?
- Participation at free will: does the researcher guarantee anonymity and confidentiality with respect to local authorities? The possibility to withdraw from the activity at any time and the absence of coercion to participate?
The platforms recommended by the institution for research purposes are the following:
- An institutional version of LimeSurvey has been approved for even highly sensitive data collection, available at: https://catalogue-si.unige.ch/en/limesurvey.
- For storage of your data, the institutional storage system is recommended: https://catalogue-si.unige.ch/en/stockage-recherche.
If I plan to use a platform that is not yet “recommended”, what prior steps should I take?
- We advise you to contact the UNIGE’s data protection officer (DPO) who will be able to evaluate the use of a cloud solution for your research purposes.
- The following form can be sent to the DPO to evaluate the cloud solution you would like to use for research purposes.
- Depending on the sensitivity of the data you plan to process, the provider and host sites, and the technical and contractual terms and conditions, you will need to obtain explicit approval from the Rectorate before using the platform. Based on your request, the DPO will be able to specify the prerequisites required by UNIGE.
18. Usage du terme “race” en recherche : questions éthiques
Le concept de “race” a une longue histoire et a été utilisé dans de nombreux contextes différents. Bien que son usage ait varié d’une époque à l’autre, il a toujours été lié à une volonté de construire et imposer des différences prétendument biologiques et/ou culturelles entre les groupes humains, et son développement dans le contexte scientifique du 19e siècle est indissociable d’une volonté de domination, de contrôle et de pouvoir, notamment en contexte colonial. Dans la seconde partie du 20ème siècle, ce concept a été remis en question à la fois sur le plan scientifique et sur le plan éthique. Les progrès de l’anthropologie et de la génétique ont montré que les différences génétiques entre les populations humaines étaient minimes, que leurs variations phénotypiques étaient continues et conditionnées par les environnements, et que les classifications raciales étaient de ce fait arbitraires et sans fondement biologique rationnel. Par ailleurs, des recherches en sciences humaines et sociales ont montré que les distinctions raciales sont socialement construites sur la base d’identités culturelles et historiques, et que les catégories changent en fonction du contexte. Aujourd’hui, le terme “race” est néanmoins encore utilisé dans le langage commun pour désigner des groupes humains sur la base de critères biologiques et/ou socio-culturels flous, ce qui accentue des stratifications sociales et engendre des discriminations. Dans ce contexte, quelles sont les recommandations éthiques liées à l’usage du concept de « race » dans une recherche scientifique ?
Dans certains pays, comme les USA, le Canada ou la Grande Bretagne, le terme « race » fait partie du langage courant et, même si le concept est contesté (1, 2), il est utilisé dans des cadres légaux (recensement, etc.). Dans d’autres pays, comme la Suisse ou la France, son usage est généralement évité au nom des valeurs d’universalisme et d’égalité (3–5). Ces restrictions d’usage font aussi polémique, dans la mesure où elles sont accusées d’empêcher la mise en évidence de faits sociaux comme la discrimination à l’embauche, ou le contrôle au faciès (6). Les chercheurs et les chercheuses peuvent donc parfois se retrouver en tension entre l’incongruité du concept de race d’un point de vue biologique, et l’évidence de sa réalité dans le monde social (7).
Ainsi, même s’il est admis que la notion de race n’a pas de sens d’un point de vue biologique, elle peut être pertinente dans le cadre de recherches qui visent, par exemple, à examiner comment des différences phénotypiques perceptibles peuvent être associées à une hiérarchisation sociale ou à des rapports interpersonnels particuliers (8).
Cependant, étant donné le sens controversé que véhicule ce mot et son caractère sensible, la CUREG examinera avec attention les justifications proposées par les chercheurs ou les chercheuses qui l’utilisent. À la suite d’une consultation d’expertes et des membres de la commission plénière de la CUREG, des préconisations ont été définies. Les projets seront examinés dans ce cadre.
- Le terme « race » ne peut être utilisé que dans un contexte où son usage est clairement justifié, par exemple lorsqu’il s’agit de mesurer le niveau d’adhésion à des thèses racistes, ou lorsque les acteurs et les actrices concerné-es s’approprient le concept dans des revendications contre toute forme de discrimination raciale.
Mais la plupart du temps, Il est conseillé de ne pas utiliser ce terme. Cela vaut pour le matériel et pour les textes associés (titre de la recherche, résumé, description du protocole, etc.).
- Il est également recommandé de ne pas utiliser de dénominations ou de catégorisations suggérant l’existence d’une typologie raciale ou renvoyant une vision réductrice de la diversité biologique et/ou culturelle humaine, qui posent les mêmes problèmes éthiques que le mot « race » ; par exemple « caucasien », « noir », « phénotype asiatique », etc.
- L’usage de termes ou expressions comme « ethnie », « appartenance ethnique », « origine », « ethnie, ethnicité et autres circonstances particulières », « origine ethnique », « origine ethnique, sociale et territoriale », « ancestralité », etc., est souvent proposé comme alternatives éthiquement correctes. Mais ces appellations ambiguës ou mal définies doivent être utilisées avec précaution et toujours en justifiant leur usage dans le cadre de la demande d’évaluation éthique.
- Lorsqu’une variable liée à l’apparence physique des participant-es est mesurée, les chercheurs et les chercheuses doivent justifier la nécessité scientifique du recueil de cette donnée. Il est recommandé de mettre la priorité sur des termes spécifiques plutôt que collectifs, et de choisir des descripteurs de même nature pour tous les individus. Par exemple, pour étudier l’effet de la « race » sur le contrôle au faciès, il est préférable de recueillir une donnée comme la couleur de peau, si possible à l’aide d’une mesure qui laisse libre cours à toute la diversité de cette caractéristique (par exemple, les palettes continues de teintes de peau existant dans le commerce).
- La mesure de l’auto-identification d’une personne à une « race » doit être évitée et, si nécessaire, remplacée par des critères d’auto-identification choisis avec beaucoup de précautions. Il n’est pas rare de lire des questions comme « quelle est votre race/ethnicité : Caucasien, etc. ». Une formulation neutre (ex : « je me décrirais comme… ») proposant une réponse ouverte est recommandée. Si des réponses fermées à choix multiples doivent être soumises, l’option « aucune des réponses ne me décrit » doit être obligatoirement présente.
Références
- J. Nicol, B. Osazuwa, Les mots pour parler de race et d’ethnicité : une terminologie en évolution. Notes de la Colline, (available at https://notesdelacolline.ca/2022/01/31/les-mots-pour-parler-de-race-et-dethnicite-une-terminologie-en-evolution/).
- Committee on the Use of Race, Ethnicity, and Ancestry as Population Descriptors in Genomics Research, Board on Health Sciences Policy, Committee on Population, Health and Medicine Division, Division of Behavioral and Social Sciences and Education, National Academies of Sciences, Engineering, and Medicine, Using Population Descriptors in Genetics and Genomics Research: A New Framework for an Evolving Field (National Academies Press, Washington, D.C., 2023; https://www.nap.edu/catalog/26902).
- P. Mahon, La notion de « race » dans le droit suisse : à jeter aux oubliettes ? TANGRAM 44 (2020), (available at https://www.ekr.admin.ch/f585.html).
- M. Merenda, La définition de la race par les autorités judiciaires suisses. TANGRAM 44 (2020), (available at https://www.ekr.admin.ch/publications/f587.html).
- C. Gombault, G. Grenet, L. Segurel, L. Duret, F. Gueyffier, P. Cathébras, D. Pontier, S. Mainbourg, A. Sanchez‐Mazas, J. Lega, Population designations in biomedical research: Limitations and perspectives. HLA. 101, 3–15 (2023).
- R. Gremaud, Les discriminations raciales ne sont pas chiffrées en Suisse. ma RTS (2020), (available at https://www.rts.ch/info/suisse/11397852-les-discriminations-raciales-ne-sont-pas-chiffrees-en-suisse.html).
- J. L. Martin, K.-T. Yeung, The Use of the Conceptual Category of Race in American Sociology, 1937–99. Sociological Forum. 18, 521–543 (2003).
- C. L. Ford, N. T. Harawa, A new conceptualization of ethnicity for social epidemiologic and health equity research. Social Science & Medicine. 71, 251–258 (2010).
19. Ma méthode de recherche ne me permet pas ou difficilement de recueillir un consentement. Comment faire ?
La CUREG a identifié deux cas pour lesquels il peut y avoir une demande de dérogation au consentement.
CAS 1 : observation sans manipulation de l’environnement.
Il s’agit des études où les chercheurs/euses observent les comportements spontanés, sans aucune intervention ni manipulation. Par exemple il peut s’agir d’une recherche où on observe la vitesse des conducteurs/trices sur une portion de route en fonction du modèle de voiture. Ou bien une étude où l’on compte le nombre de prises de parole dans un cours en fonction du sexe des élèves. Dans ce cas, la CUREG peut examiner une demande argumentée d’absence de consentement, aux conditions suivantes :
– L’étude est anonyme dès le recueil des données (pas de voix, vidéo, photo, etc.).
– L’observation se fait en cadre naturel (situation quotidienne, publique, espace public environnement usuel) ou lieu public (p. ex. stade, bibliothèque, musée, planétarium, plage ou parc) ou dans un environnement numérique (p. ex. groupes en ligne) ou dans des espaces privés ou protégés (p. ex. clubs privés, clubs sportifs ou organisations).
– Le chercheur ou la chercheuse n’est pas intervenu-e sur la situation.
– Il n’y a pas de conflit d’intérêts.
CAS 2 : observation avec manipulation de l’environnement à l’insu des personnes.
Il s’agit d’observations en situation naturelle, mais le chercheur ou la chercheuse induit une manipulation de l’environnement afin d’en tester les effets. Par exemple, une étude pourrait tester différentes formes de courriers d’invitation au don du sang, afin de voir laquelle est la plus efficace. Ou bien, des spécialistes en comportements environnementaux pourraient tester différents messages pour inciter les usagers d’un bâtiment à utiliser les escaliers plutôt que les ascenseurs. Dans ce cas, la CUREG peut examiner une demande argumentée d’absence de consentement, aux conditions suivantes :
– L’étude est anonyme dès le recueil des données (pas de voix, vidéo, photo, etc.).
– L’observation se fait en cadre naturel (situation quotidienne, publique, espace public environnement usuel) ou dans un lieu public (p. ex. stade, bibliothèque, musée, planétarium, plage ou parc) ou dans un environnement numérique (p. ex. groupes en ligne) ou dans des espaces privés ou protégés (p. ex. clubs privés, clubs sportifs ou organisations).
– Il n’y a pas de risques induits par la manipulation.
– Il n’y a aucune forme de consentement possible, a priori ou a posteriori, sans mettre en péril la recherche.
– La prise de risques éthique est justifiée par les enjeux de la recherche.
– Il n’y a pas de conflit d’intérêts.
Dans tous les cas, lorsque cela est possible, les chercheurs et les chercheuses sont invité-es à prendre des mesures pour informer le public concerné qu’une recherche est en cours et de diffuser le nom et les coordonnées de la personne responsable (par voie d’affichage ou de documents distribués lors de l’achat des tickets d’entrée par exemple).
20. Pourquoi la CUREG me demande de m’adresser à la Commission Cantonale d’Éthique de la Recherche ?
Les règles fédérales Suisses, reposant sur la loi sur la Recherche sur l’Humain (LRH) et son application, imposent aux chercheurs et aux chercheuses de passer par la CCER dans les cas suivants :
- recherche relative aux causes et aux fondements des maladies, y compris les aspects psychosociaux[1].
- les études portant sur la mesure de l’activité cérébrale (IRM, EEG, etc.) ou de la physiologie périphérique (ECG, mesure de l’activité électrodermale, etc.) durant une observation expérimentale du comportement (scénario Y de la prise de position swissethics[2]).
- les essais cliniques (interventions)[3].
Parfois, même si votre recherche tombe dans l’une de ces situations, la CCER considère qu’elle ne souhaite pas entrer en matière. Dans ce cas, la CUREG accepte de traiter votre dossier. Mais vous devez fournir une attestation de non entrée en matière de la part de la CCER. Pour des raisons juridiques, cette attestation doit être fournie pour chaque nouveau projet soumis.
[1] Message sur la loi fédérale relative à la recherche sur l’être humain du 21 octobre 2009, section 1.8.1.3 p. 7294.
[2] Guide de swissethics : demandes dans le domaine des neurosciences du 14 janvier 2016
[3] Ordonnance sur les essais cliniques hors essais cliniques de dispositifs médicaux du 20 septembre 2013
21. Recherche à visée de formation : dois-je soumettre à la CUREG ?
Certaines situations impliquent un recueil de données auprès de participant-es humain-es, mais avec un objectif de formation uniquement (par exemple, maîtriser une technique de recueil de donnée ou avoir une expérience du processus de recherche) et sans visées scientifiques. Cela peut être le cas de travaux pratiques ou de travaux de recherches en Bachelor. Dans ces cas une attestation éthique n’est pas nécessaire et la CUREG n’entre pas en matière. Les aspects éthiques sont sous la responsabilité des enseignant-es. Si ces travaux s’adossent à un projet de recherche plus large, celui-ci doit être traité dans le cadre de la procédure de dépôt de projets.
22. J’utilise des applications, des logiciels ou des plateformes externes à l’UNIGE pour recueillir mes données (Qualtrics, RealLife Exp, Gorilla, Prolific, Field Notes, etc.), quelles précautions dois-je prendre ?
Le recours à des outils externes à l’UNIGE pour recueillir des données de recherche est devenu très courant. Cette pratique pose trois problèmes potentiels : 1) la conformité légale du stockage des données 2) l’usage potentiel des données par le fournisseur de l’outil et 3) le consentement éclairé des participant-es.
- Conformité légale. Compte-tenu de la rapidité du développement des produits, les services internes de l’UNIGE ne peuvent pas valider l’usage de tous les outils offerts aux chercheurs et aux chercheuses. Il relève donc de votre responsabilité de vérifier la compatibilité de celui que vous utilisez avec les lois en vigueur en Suisse (notamment la LIPAD). Cette information se trouve généralement de façon déclarative dans les politiques de confidentialité de votre fournisseur.
- Usage des données par le fournisseur. Une conformité légale peut être compatible avec l’utilisation des données recueillies par le fournisseur à des fins commerciales. Cela peut aller de la simple utilisation pour améliorer l’outil, à la commercialisation des données fournies par vos participant-es, par exemple pour entraîner des intelligences artificielles. Il vous appartient de vérifier les pratiques de votre fournisseur.
- Consentement des participant-es. Si le fournisseur peut utiliser les données des participant-es (même de façon anonyme et agrégée) à d’autres fins que celles de votre recherche, les participant-es doivent en être informé-es à l’aide du formulaire de consentement. Une phrase type pourrait être « Nous utilisons une application tierce pour recueillir vos réponses. Notre fournisseur peut utiliser ces données [le cas échéant préciser : de façon anonyme et agrégée] à des fins privées [éventuellement préciser lesquelles]. »
23. I conduct research within an organization: what is the difference between the permission obtained from the organization’s authorities and the consent of the participants?
Distinction between Entry Agreement and Participant Consent
When conducting research with human participants within an organization (business, institution, association, school, etc.), it is often necessary to obtain the agreement or authorization from a person with authority in that organization (a person holding the status of “gatekeeper”).
The agreement to conduct research within an organization does not imply the consent of the members of that organization to participate in the research. The term “consent” implies the explicit agreement of the participants in the study; an organization cannot give consent on behalf of the participants. The Swiss Academy of Sciences’ Code of Scientific Integrity defines it as follows:
“Informed consent is a procedure by which a researcher obtains and maintains permission from a person, or their legal representative, for that person to participate in a research study” (ASS, 2020, p. 13).
Who are the Participants? Who Consents?
The participants, in other words, the individuals from whom consent should be obtained, are the people from whom data is collected.
In research conducted within an organization, the agreement is therefore not sufficient, and obtaining the consent of each participant is necessary (except in special cases, see FAQ-19).
A Suggestion for Further Reading:
Canadian Code “Ethics of Research Involving Humans”, Tri-Council Policy Statement (TCPS 2), 2022, https://ethics.gc.ca/fra/policy-politique_tcps2-eptc2_2022.html